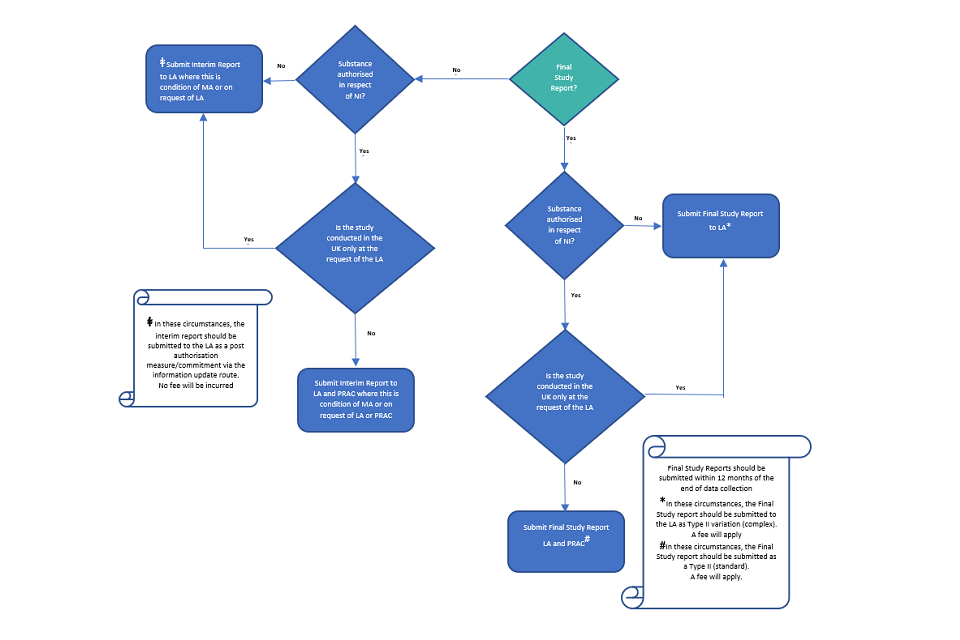
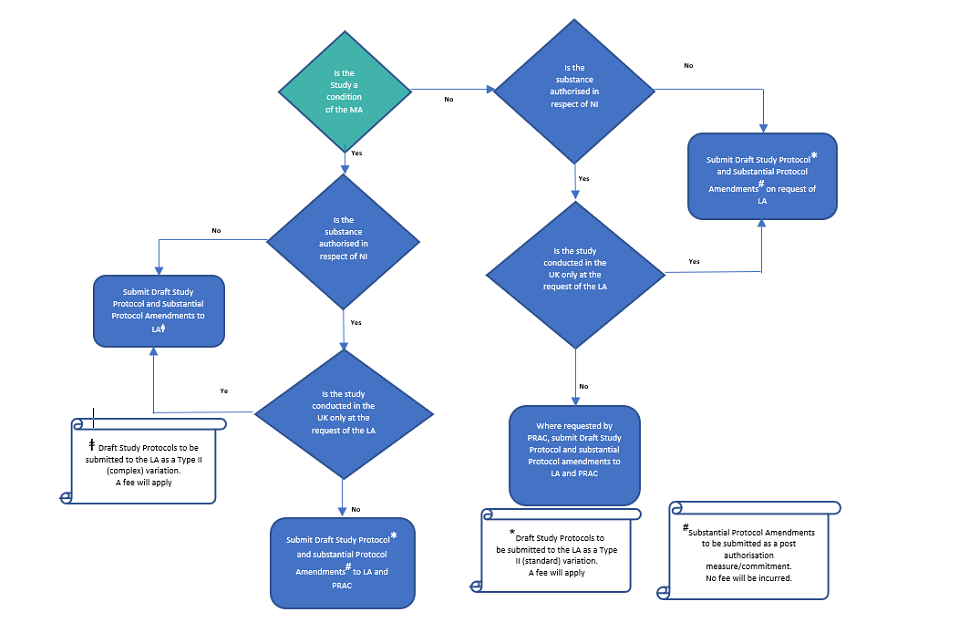
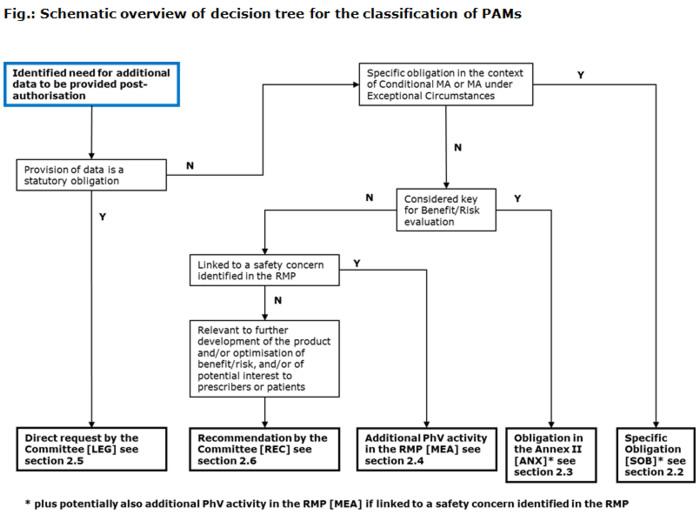
EU Regulatory Pathways for ATMPs: Standard, Accelerated and Adaptive Pathways to Marketing Authorisation - ScienceDirect

Janssen seeks EMA approval for single tablet pulmonary arterial hypertension treatment - Pharmaceutical Technology

EU Regulatory Pathways for ATMPs: Standard, Accelerated and Adaptive Pathways to Marketing Authorisation: Molecular Therapy Methods & Clinical Development

CONSORT flow chart of the prospective cohort study. EMA ¼ endovenous... | Download Scientific Diagram

EU Regulatory Pathways for ATMPs: Standard, Accelerated and Adaptive Pathways to Marketing Authorisation: Molecular Therapy Methods & Clinical Development
CONSORT flow chart of the prospective cohort study. EMA ¼ endovenous... | Download Scientific Diagram